Synthesis, properties and reactivity of BCl2 aza-BODIPY complexes and salts of the aza-dipyrrinato scaffold†
Abstract
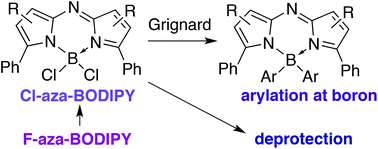
The synthesis and characterisation of the BCl2-chelated complexes of both archetypal aza-dipyrrin sub-types are presented. A stepwise halogen exchange, leading to a mixed-halide Cl–B–F intermediate, is implicated in the conversion of F-aza-BODIPYs to Cl-aza-BODIPYs upon treatment with BCl3. The utility of the Cl-aza-BODIPY scaffold to facilitate substitutions at boron is demonstrated under mild conditions through treatment with aryl Grignard reagents. Additionally, the lability of the B–Cl bond enables facile removal of the BCl2 group, i.e. deprotection of F-aza-BODIPYs, under aqueous conditions. Three aza-dipyrrin HX salts were also synthesised and characterised. The pKa of the protonated aza-dipyrrin was determined to be 4, thereby providing insight regarding the storage and stability of such species.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...