A silver-catalyzed radical ring-opening reaction of cyclopropanols with sulfonyl oxime ethers†
Abstract
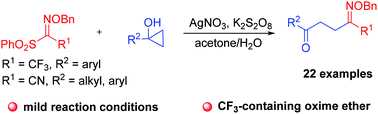
A silver-catalyzed ring-opening reaction of cyclopropanols with sulfonyl oxime ethers has been developed. The protocol was conducted under mild reaction conditions to provide a series of γ-keto oxime ethers with moderate to good yields. The reaction proceeded in a stereoselective manner for CF3-containing oxime ethers to provide a single stereoisomer, while an inseparable E and Z mixture was obtained for CN-containing oxime ethers. Mechanistic studies indicate that the reaction proceeded via a radical mechanism.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...