Copper(i)/Ganphos catalysis: enantioselective synthesis of diverse spirooxindoles using iminoesters and alkyl substituted methyleneindolinones†
Abstract
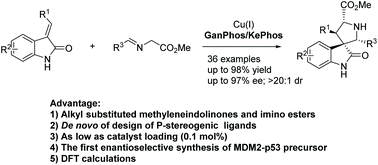
A copper-catalyzed asymmetric 1,3-dipolar cycloaddition of glycine iminoesters with alkyl substituted 3-methylene-2-oxindoles is described. By using de novo design of P-stereogenic phosphines as ligands, spiro[pyrrolidin-3,3′-oxindole]s are generated in good to excellent yields with high asymmetric induction. A further reduced catalyst loading of 0.1 mol% is sufficient to achieve a satisfactory enantioselectivity of 90% ee. The DFT calculations suggest the second Michael addition of the 1,3-dipole to be the rate- and enantio-determining step. A key feature of this 1,3-dipolar cycloaddition is the wide substrate applicability, even with alkyl aldehyde-derived azomethine ylide; thus it has streamlined a highly enantioselective access to a new class of antiproliferative agents, MDM2-p53.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...