Copper(i)-catalyzed benzylation of triazolopyridine through direct C–H functionalization†
Abstract
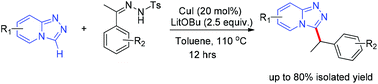
A general and efficient copper-catalyzed benzylation reaction of triazolopyridine with N-tosylhydrazones was developed. This reaction forms a C(sp2)–C(sp3) bond through cross-coupling, and represents an exceedingly practical method to afford 3-benzylated triazolopyridines in moderate to good yields. A proposed mechanistic pathway underlying this reaction was outlined. This catalytic transformation should enable broad synthetic applications in functionalization chemistry, allowing the synthesis of new pharmaceutically relevant triazolopyridine derivatives.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...