α-2′-Deoxyguanosine can switch DNA G-quadruplex topologies from antiparallel to parallel†
Abstract
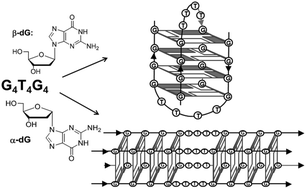
Here we demonstrate G-quadruplex formation by oligodeoxynucleotides containing α-2′-deoxyguanosine (α-dG) as a sole source of guanosines in G4T4, G4T4G4 and T(G3Tn)3G3T sequences with various numbers of natural β-T in the loops (n = 1–4). Based on circular dichroism spectra we observed that all α-dG-containing DNAs formed G-quadruplexes with uniform arrangement of α-dG-tetrads, which implies formation of G-quadruplexes of parallel topology. In several cases, native DNA structures that usually adopt an antiparallel topology were converted to more thermally stable G-quadruplexes of parallel topology. Using 2D ROESY NMR spectra a new ‘sequential walk’ was established for α-dGs in a tetramolecular, parallel complex formed by the α-G4β-T4 sequence. Analysis of ROEs in α-dGs indicates that guanines in [α-G4β-T4]4 adopt anti-glycosidic conformations. These results demonstrate that α-dG can be used for an antiparallel-to-parallel switch of G-quadruplex DNAs producing complexes with higher thermal stability and uniform stacking of α-dG-tetrads.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...