The pH-influenced PET processes between pyronine and different heterocycles†
Abstract
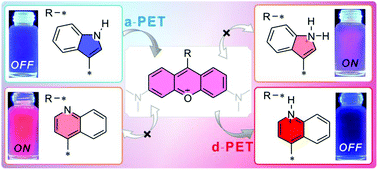
The OFF–ON and ON–OFF type pH probes based on rosamine were designed by using the relative electron densities between pyronine and various linked heterocycles. Probe 1a with an indole–pyronine skeleton gave an OFF–ON pH response (pKa = 1.41) with decreasing pH, and the relative fluorescence intensity increased 15-fold, while probe 1b with an imidazole–pyronine skeleton did not give an ON–OFF response to different pH values. When pyronine was connected with a quinolinyl group, i.e., probes 1c–d, the red emission (around 575–800 nm) gave a monotonous ON–OFF pH response (pKa = 3.26 and 2.62, respectively) with decreasing pH. The relative fluorescence intensities decreased 263- and 46-fold, respectively. Changes in the electron donating abilities of the nitrogen containing heterocycles were used to explain variations in PET processes within the probes, and their pH-dependent PET mechanisms were verified using time-dependent density functional theory calculations. Confocal fluorescence imaging was also used to evaluate the potential biomedical application of probes 1a–d. Ultimately, probe 1d with an appropriate pKa value and good biocompatibility showed lysosome targeting ability.


 Please wait while we load your content...
Please wait while we load your content...