B(C6F5)3 catalysed reduction of para-quinone methides and fuchsones to access unsymmetrical diaryl- and triarylmethanes: elaboration to beclobrate†
Abstract
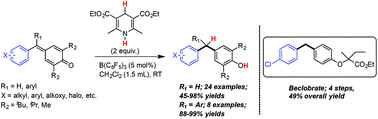
A mild and efficient method for the synthesis of unsymmetrical diaryl- and triarylmethanes through a B(C6F5)3 catalyzed reduction of para-quinone methides and fuchsones respectively, using the Hantzsch ester as a reducing source has been developed. Detailed mechanistic investigations revealed that the reaction actually proceeds through a Lewis acid–base pair complex derived from B(C6F5)3 and the Hantzsch ester.


 Please wait while we load your content...
Please wait while we load your content...