Enantioselective isothiourea-catalysed trans-dihydropyridinone synthesis using saccharin-derived ketimines: scope and limitations†
Abstract
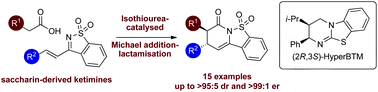
The catalytic enantioselective synthesis of a range of trans-dihydropyridinones from aryl-, heteroaryl- and alkenylacetic acids and saccharin-derived ketimines with good to excellent stereocontrol (15 examples, up to >95 : 5 dr, up to >99 : 1 er) is reported. After extensive optimisation, HyperBTM proved the optimal isothiourea catalyst for this transformation at −78 °C, giving trans-dihydropyridones with generally excellent levels of diastereo- and enantioselectivity.

 Please wait while we load your content...
Please wait while we load your content...