Syntheses of pterocarpenes and coumestans via regioselective cyclodehydration†
Abstract
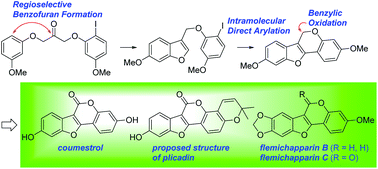
A highly efficient synthetic route to pterocarpenes and coumestans is described. BCl3-mediated dehydrative cyclization of 1,3-diaryloxyacetones under mild conditions permitted regioselective ring closure to afford 3-((2-iodoaryloxy)methyl)benzofurans which were converted to the corresponding pterocarpenes by Pd-catalyzed intramolecular direct arylation. The subsequent benzylic oxidation led to coumestans. This sequence was applied to the formal syntheses of coumestrol and the proposed structure of plicadin as well as total syntheses of flemichapparins B and C.

 Please wait while we load your content...
Please wait while we load your content...