Metal-free oxidative amidation of aldehydes with aminopyridines employing aqueous hydrogen peroxide†
Abstract
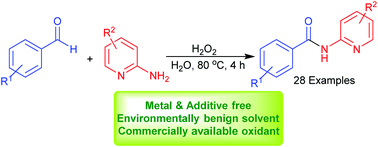
The first metal free report on the amidation of aldehydes with aminopyridines was accomplished using simple aqueous hydrogen peroxide (aq. H2O2) as the oxidant. No catalysts or additives were needed for this transformation and the reaction proceeded in water, an environmentally benign reaction medium. Green oxidant and reaction conditions, and the ability to construct diverse N-(pyridin-2-yl)benzamide by this elegant method render it a practical alternative for the synthesis of these amides.


 Please wait while we load your content...
Please wait while we load your content...