Highly efficient anion transport mediated by 1,3-bis(benzimidazol-2-yl)benzene derivatives bearing electron-withdrawing substituents†
Abstract
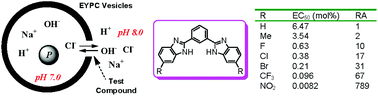
1,3-Bis(benzimidazol-2-yl)benzene exhibits potent anionophoric activity through a process of anion exchange with a minor level of proton/anion symport. Modification of 1,3-bis(benzimidazol-2-yl)benzene with strong electron-withdrawing substituents, such as trifluoromethyl and nitro groups, leads to up to 789-fold increase in the activity. The benzimidazolyl–NH fragments, the relative position and the number of the benzimidazolyl groups on the central phenyl scaffold play an essential role in the transport.

 Please wait while we load your content...
Please wait while we load your content...