Chemoselective acylation of benzimidazoles with phenylacetic acids under different Cu catalysts to give fused five-membered N-heterocycles or tertiary amides†
Abstract
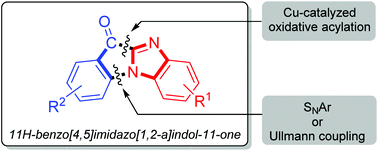
C–N bond formation via a copper-catalyzed aerobic oxidative decarboxylative tandem protocol was realized. The phenylacetic acids which contain ortho-X (X = F or Br) on the aromatic ring will render a fused five-membered heterocycle via a tandem aromatic nucleophilic substitution and aerobic oxidative decarboxylative acylation at the C(sp2)–H bond of benzimidazoles under the Cu(OAc)2/K2CO3/BF3·Et2O catalytic system, while with CuBr as the catalyst and pyridine as the base, N-acylation occurred and tertiary amides were obtained.


 Please wait while we load your content...
Please wait while we load your content...