A ThDP-dependent enzymatic carboligation reaction involved in Neocarazostatin A tricyclic carbazole formation†
Abstract
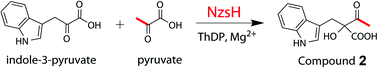
Although the biosynthetic pathway of Neocarazostatin A (1) has been identified, the detailed enzymatic reactions underlying the assembly of the carbazole ring still remain largely unknown. We demonstrate here that NzsH, a putative thiamine diphosphate dependent enzyme, can catalyze an acyloin coupling reaction between indole-3-pyruvate and pyruvate to generate a β-ketoacid intermediate. Our findings thus shed light on further characterization of the unusual biosynthetic pathway of the bacterial tricyclic carbazole alkaloids.

 Please wait while we load your content...
Please wait while we load your content...