Electronic effects on the substitution reactions of benzhydrols and fluorenyl alcohols. Determination of mechanism and effects of antiaromaticity†
Abstract
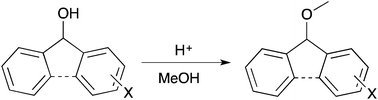
A range of substituted benzhydrols and fluorenols were prepared and subjected to acid catalysed methanolysis. Analysis of the rates of each of these processes showed correlation with Hammett σ+ parameters as is consistent with the significant build-up of positive charge adjacent to the ring. In combination with the similarity of the electronic susceptibility of the processes, these data suggest that both reactions proceed through a unimolecular rate-determining step. This shows that the effect of fusion of the phenyl systems (and hence potentially introducing an antiaromatic carbocation intermediate) is only to slow the rate of reaction rather than change the mechanism of the process.

 Please wait while we load your content...
Please wait while we load your content...