Origins of observed reactivity and specificity in the addition of B2Cl4 and analogues to unsaturated compounds†
Abstract
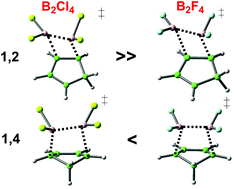
In 1954 Schlesinger and co-workers observed the direct reaction of diboron tetrachloride with simple organic compounds under mild conditions, the 1,2 addition product being formed with either ethylene or acetylene. In the following 25 years a series of addition reactions to simple alkenes, alkynes and dienes was demonstrated. B2F4 was shown to react in similar manner, albeit under more forcing conditions. Crucially, it was demonstrated that the addition to (E)- or (Z)-but-2-ene occurred with cis-stereospecificity. Only sporadic interest was shown in this field thereafter until catalysed addition reactions of diboron reagents were realized. Encouraged by this revival of interest through the discovery of transition-metal and nucleophilic catalysis of diboryl additions, DFT analysis of uncatalysed additions of B2X4 has been carried out and interpreted. This includes the relative reactivity of several B–B reagents with ethene, and that of B2Cl4vs. B2F4 additions, including benzene, naphthalene and C60 as reactants. This allows the analysis of relative reactivity vis-à-vis substitution on boron, and also direct comparison with hydroboration by HBCl2. [4 + 2] Addition of diboron reagents to dienes with B–B cleavage competes with direct [2 + 2] addition, favourably so for B2F4. The computational results demonstrate that the stereospecific addition to isomeric but-2-enes is a rare concerted [2σs + 2πs] process.

 Please wait while we load your content...
Please wait while we load your content...