Isolation and structural determination of non-racemic tertiary cathinone derivatives†
Abstract
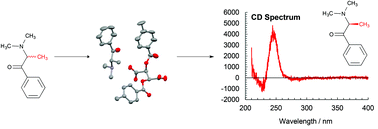
The racemic tertiary cathinones N,N-dimethylcathinone (1), N,N-diethylcathinone (2) and 2-(1-pyrrolidinyl)-propiophenone (3) have been prepared in reasonable yield and characterized using NMR and mass spectroscopy. HPLC indicates that these compounds are isolated as the anticipated racemic mixture. These can then be co-crystallized with (+)-O,O′-di-p-toluoyl-D-tartaric, (+)-O,O′-dibenzoyl-D-tartaric and (−)-O,O′-dibenzoyl-L-tartaric acids giving the single enantiomers S and R respectively of 1, 2 and 3, in the presence of sodium hydroxide through a dynamic kinetic resolution. X-ray structural determination confirmed the enantioselectivity. The free amines could be obtained following basification and extraction. In methanol these are reasonably stable for the period of several hours, and their identity was confirmed by HPLC and CD spectroscopy.


 Please wait while we load your content...
Please wait while we load your content...