Investigation of the active turn geometry for the labour delaying activity of indolizidinone and azapeptide modulators of the prostaglandin F2α receptor†
Abstract
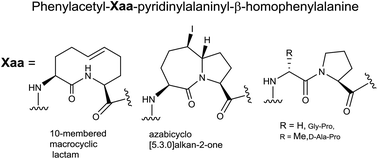
On pursuing molecules that delay labour, so-called tocolytics, the prostaglandin F2α receptor (FP) was targeted, because of its role in the stimulation of uterine contractions leading to birth and preterm birth. Previously, both the indolizidinone PDC-113.824 (5) and the aza-glycinyl-proline analog 6 were shown to delay labour in mice by modulating the FP function, likely by an allosteric mechanism, which features biased signalling. The crystal structure and computational analyses of the indolizidin-2-one amino acid and aza-glycinyl-proline components of 5 and 6 in model peptides have shown them to adopt a geometry that mimics ideal type I and II′ β-turns. To elucidate the precise turn geometry for receptor recognition, analogs 1–4 have now been synthesized: macrocycle and pyrroloazepinone mimics 1 and 2 to mimic type I, and glycinyl-proline and D-alaninyl-proline analogs 3 and 4 to favour type II′ β-turn geometry. Notably, transannular cyclization of peptide macrocycle 13 has provided diastereoselectively pyrroloazepinone 15 by a novel route that provides effective access to mimics 1 and 2 by way of a common intermediate. Among the four analogs, none exhibited efficacy nor potency on par with 5 and 6; however, D-alaninyl-proline analog 4 proved superior to the other analogs in reducing PGF2α-induced myometrial contractions and inhibiting FP modulation of cell ruffling, a response dependent on the Gα12/RhoA/ROCK signaling pathway. Furthermore Gly-Pro analog 3 potentiated the effect of PGF2α on Gαq mediated ERK1/2 activation. Evidence that 4 adopted turn geometry was obtained by conformational analysis using NMR spectroscopy to characterize respectively the influence of solvent and temperature on the chemical shifts of the amide NH protons. Although mimicry of the type II′ geometry by 3, 4, 5 and 6 may favour activity, distortion from ideal geometry by the indolizidinone and aza-glycinyl residues of the latter appears to enhance their biological effects.

 Please wait while we load your content...
Please wait while we load your content...