The first asymmetric total synthesis of lycoposerramine-R†
Abstract
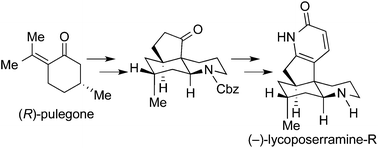
The first asymmetric total synthesis of lycoposerramine-R, a Lycopodium alkaloid possessing a novel skeleton, was accomplished by a strategy featuring the stereoselective intramolecular aldol cyclization giving a cis-fused 5/6 bicyclic skeleton and a new method for the construction of the pyridone ring via the aza-Wittig reaction.

 Please wait while we load your content...
Please wait while we load your content...