Double-headed nucleotides introducing thymine nucleobases in the major groove of nucleic acid duplexes†
Abstract
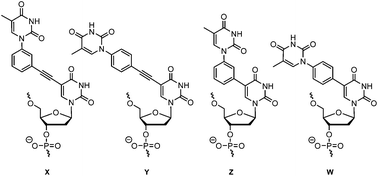
Four different double-headed nucleosides each combining two thymine nucleobases with different linkers were synthesised. The 5-position of 2′-deoxyuridine was connected to the N1-position of a thymine through either m- or p-disubstituted phenyl or phenylacetylene linkers by the use of Suzuki or Sonogashira couplings. When introduced into oligonucleotides, the thermal stability of dsDNA and DNA : RNA duplexes were determined and structural information was obtained from CD- and fluorescence spectroscopy. Also the recognition of abasic sites was studied. In general, the more stable duplexes were obtained with m- rather than p-substitution and with phenylacetylene rather than phenyl linkers.

 Please wait while we load your content...
Please wait while we load your content...