Rhodium(iii)-catalyzed cascade oxidative annulation reactions of aryl imidazolium salts with alkynes involving multiple C–H bond activation†
Abstract
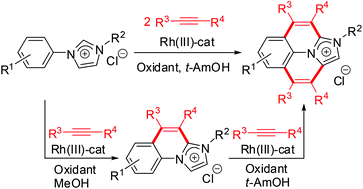
The cascade oxidative annulation reactions of aryl imidazolium salts with alkynes proceed efficiently in the presence of [Cp*RhCl2]2 and Cu(OAc)2·H2O to give substituted imidazo[1,2-a]-quinolinium salts and benzo[ij]imidazo[2,1,5-de]quinolizinium salts. The reactions were through the normal and abnormal N-heterocyclic carbene (NHC)-directed cyclometalation, alkyne insertion into the Rh–C bond, and reductive elimination of alkenyl and NHC ligands. The reactions are highly regioselective with unsymmetrical alkynes and can be achieved stepwise by controlling the reaction conditions. This provides a new application of NHCs as directing groups and substrates in the synthesis of fused N-heterocyclic compounds. The N-substituting group of the benzo[ij]imidazo[2,1,5-de]quinolizinium salts could be removed successfully with pyridine to afford benzo[ij]imidazo[2,1,5-de]quinolizines in excellent yields. Moreover, some of the benzo[ij]imidazo[2,1,5-de]quinolizinium salts exhibit intense fluorescence which might be useful in organic electronic materials.

 Please wait while we load your content...
Please wait while we load your content...