Heparan sulfate phage display antibodies recognise epitopes defined by a combination of sugar sequence and cation binding
Abstract
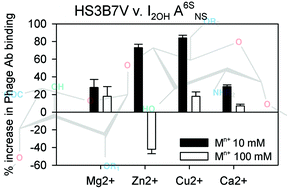
Phage display antibodies are widely used to follow heparan sulfate (HS) expression in tissues and cells. We demonstrate by ELISA, that cations alter phage display antibody binding profiles to HS and this is mediated by changes in polysaccharide conformation, demonstrated by circular dichroism spectroscopy. Native HS structures, expressed on the cell surfaces of neuroblastoma and fibroblast cells, also exhibited altered antibody binding profiles following exposure to low mM concentrations of these cations. Phage display antibodies recognise conformationally-defined HS epitopes, rather than sequence alone, as has been assumed, and resemble proteins in being sensitive to changes in both charge distribution and conformation following binding of cations to HS polysaccharides.
- This article is part of the themed collection: Multivalent Biomolecular Recognition

 Please wait while we load your content...
Please wait while we load your content...