Selective chemical modification of DNA with alkoxy- and benzyloxyamines†
Abstract
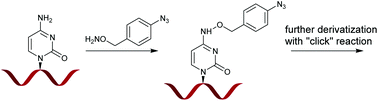
A new method for the selective chemical modification of DNA at cytosine nucleobases using alkoxy- and benzyloxyamines is presented. It is shown that in particular benzyloxyamines are effective DNA modifying agents, giving rise to almost exclusive formation of the mono addition products. By using a bifunctional derivative, that is, p-azidobenzyloxyamine hydrochloride, an azide moiety, which is a convenient handle for further functionalization, could be introduced into the DNA. The azido modified DNA was then further reacted in a copper(I)-monophos catalysed 1,3-dipolar cycloaddition. These results illustrate the potential of the presented method for application in site and chemo-selective modification of DNA.

 Please wait while we load your content...
Please wait while we load your content...