Gold-catalysed glycosylation reaction using an easily accessible leaving group†
Abstract
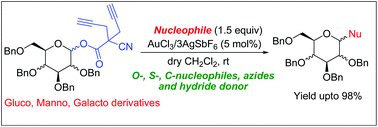
Gold(III)-catalysed glycosylation reaction has been developed by employing a new and easily accessible leaving group synthesized from ethyl cyanoacetate. Several nucleophiles like alcohols, thiols, allyltrimethylsilane, trimethylsilyl azide and triethylsilane have been reacted to make the corresponding glycosides in good yields and with marginal to excellent α-selectivity.

 Please wait while we load your content...
Please wait while we load your content...