BF3-Et2O mediated skeletal rearrangements of norbornyl appended cyclopentanediols†
Abstract
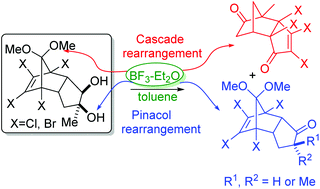
An unusual cascade rearrangement has been noticed as a competitive reaction during the treatment of norbornyl appended cyclopentanediols with a Lewis acid (LA): a BF3-Et2O mediated pinacol–pinacolone rearrangement. Deketalization and pinacolone rearrangement occur at two different sites in the molecule and are responsible for the observed cascade rearrangement product. However, deketalization appears to be triggering the cascade steps. The kinetically more stable pinacolone product with an exo-Me group was observed in the case of the bromo analogue, whereas, the thermodynamically more stable pinacolone product with an endo-Me group was observed in the case of the chloro analogue. Epimerization via tautomerization of one diastereomer to the other diastereomer under Lewis acid reflux conditions is possible. On the contrary, the diol equivalent epoxides provide only the diastereomeric mixture of pinacolone products under similar LA reaction conditions. The lower yields observed in the case of the epoxides are due to unwanted side reactions taking place between the two competitive reactive centers, namely, ketal and epoxide. Further, a sequence of elimination, nucleophilic substitution and Ritter type hydrolysis reactions of the epoxides resulted in unexpected elimination products. This transformation not only facilitates a regioselective epoxide opening, but also provides a new route for the preparation of allylic amides of the norbornyl appended cyclopentane ring system.

 Please wait while we load your content...
Please wait while we load your content...