TEMPO-mediated homocoupling of aryl Grignard reagents: mechanistic studies†
Abstract
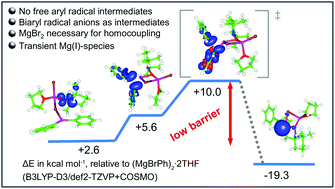
The mechanism of the TEMPO mediated oxidative homo-coupling of aryl Grignard reagents is investigated in detail by experimental and computational studies. Experimental data reveal that the nitroxide-mediated homocoupling reaction of aryl Grignard reagents does not occur via free aryl radicals. Evidence for the presence of biaryl radical anions as intermediates in the coupling reaction is provided. It is also shown that PhMgPh under bromide free conditions in the presence of TEMPO does not undergo homocoupling. However, upon addition of MgBr2, C–C bond formation smoothly proceeds documenting the important role of the bromide anions in the oxidative homocoupling. DFT calculations show that an intramolecular electron transfer to a Mg-complexed TEMPO ligand with subsequent biaryl formation in a dimeric complex is viable and in agreement with experimental reaction conditions.

 Please wait while we load your content...
Please wait while we load your content...