Ruthenium-catalyzed direct C3 alkylation of indoles with α,β-unsaturated ketones†
Abstract
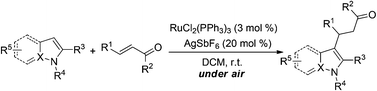
In this paper, a simple and highly efficient ruthenium-catalyzed direct C3 alkylation of indoles with various α,β-unsaturated ketones without chelation assistance has been developed. This novel C–H activation methodology exhibits a broad substrate scope such as different substituted indoles, pyrroles, and other azoles. Further synthetic applications of the alkylation products can lead to more attractive 3,4-fused tricyclic indoles.

 Please wait while we load your content...
Please wait while we load your content...