Kinetics of reactions at an interface: functionalisation of silicate glass with porphyrins via covalent bonds
Abstract
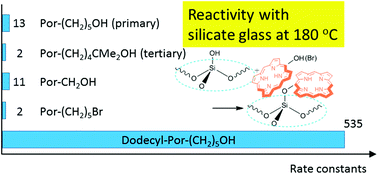
Porphyrins carrying either a primary alcohol, a tertiary alcohol or a primary bromide linker group were allowed to react with the surface silanol groups on silicate glass thermally at 80–240 °C to obtain a monolayer film. The kinetics of the reaction was analysed based on the pseudo-second order equation. The tertiary alcohol and the primary bromide reacted much slower than the primary alcohol. Arrhenius plots indicated that higher activation energies can account for the slower reaction of both tertiary alcohol and primary bromide linkers. The introduction of six dodecyl chains into hydroxyporphyrin accelerated the anchoring reaction by a factor of 50 owing to the larger frequency factor of the reaction, demonstrating that the dynamics of the interface is one of the dominant factors regulating the reaction kinetics.
- This article is part of the themed collection: Recognition and Reactivity at Interfaces

 Please wait while we load your content...
Please wait while we load your content...