A highly diastereoselective Friedel–Crafts reaction of indoles with isatin-derived N-sulfinyl ketimines towards the efficient synthesis of chiral tetrasubstituted 3-indolyl-3-aminooxindoles†
Abstract
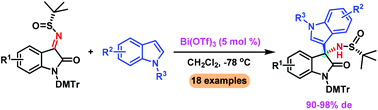
A Lewis acid promoted highly diastereoselective asymmetric Friedel–Crafts alkylation of indoles with isatin-derived N-tert-butanesulfinyl ketimines is described. The reaction can be accomplished with ease in the presence of a catalytic amount of Bi(OTf)3, providing a series of 3-indolyl-3-aminooxindoles in excellent diastereoselectivities (up to 98% de) and yields (up to 99%).

 Please wait while we load your content...
Please wait while we load your content...