l-Valine derived chiral N-sulfinamides as effective organocatalysts for the asymmetric hydrosilylation of N-alkyl and N-aryl protected ketimines†
Abstract
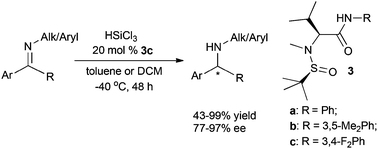
L-Valine derived N-sulfinamides have been developed as efficient enantioselective Lewis basic organocatalysts for the asymmetric reduction of N-aryl and N-alkyl ketimines with trichlorosilane. Catalyst 3c afforded up to 99% yield and 96% ee in the reduction of N-alkyl ketimines and up to 98% yield and 98% ee in the reduction of N-aryl ketimines.

 Please wait while we load your content...
Please wait while we load your content...