Breaking the dichotomy of reactivity vs. chemoselectivity in catalytic SN1 reactions of alcohols†
Abstract
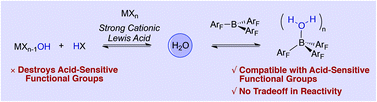
The inability to decouple Lewis acid catalysis from undesirable Brønsted acid catalysed side reactions when water or other protic functional groups are necessarily present has forced chemists to choose between powerful but harsh catalysts or poor but mild ones, a dichotomy that restricts the substrate scope of dehydrative transformations such as the direct SN1 reaction of alcohols. A systematic survey of Lewis and Brønsted acids reveals that the strong non-hydrolyzable Lewis acid B(C6F5)3 leads to highly chemoselective alcohol substitution in the presence of acid-sensitive alkenes, protecting groups and other functional groups without the typical compromise in reaction rates, substrate scope and catalyst loading.

 Please wait while we load your content...
Please wait while we load your content...