Electrochemical behaviour of new dimeric esters and amides derived from caffeic acid in dimethylsulfoxide†
Abstract
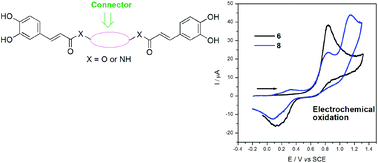
The electrochemical oxidation in DMSO of four new derivatives of caffeic acid (CA), two dimeric amides and two dimeric esters, is reported in this article. Although all of them contain two caffeoyl electroactive moieties in their structures, small differences in the connectors result in interesting changes in the electrochemical behaviour of this type of compound. Voltammograms of both esters do not show appreciable differences between them; however, an electrografting process occurs during the electrochemical oxidation of one of them, which suggests that the identity of the connector has an influence on the ability of the diesters to interact with the electrode surface. On the other hand, voltammograms of dimeric amides were more complex than those corresponding to dimeric esters. Electronic effects of diamine connectors seem to be related to the fact that caffeoyl moieties suffer from separate oxidation processes in both compounds. In contrast to their ferulic acid (FA) analogues, which have been studied by our group before, CA dimeric amides do not interact in an appreciable way with the electrode surface. In addition, a relationship between the oxidation potential and the inhibition percentage of the DPPH (2,2′-diphenyl-1-picrylhydrazyl) radical was not observed for the symmetrical CA derivatives studied here. However, the molecular flexibility seems to play a very important role in the Free Radical Scavenging Activity (FRSA) of this type of compound.

 Please wait while we load your content...
Please wait while we load your content...