Lewis acid-promoted cyclization/halogenation of allenyl ethenetricarboxylates and the corresponding amides: stereoselective synthesis of haloalkenyl five-membered heterocycles†
Abstract
Lewis acid-promoted intramolecular reactions of allenyl ethenetricarboxylates and the corresponding amides have been examined. Reactions of allenyl ethenetricarboxylates and the amides with Lewis acids such as AlCl3, AlBr3 and ZnX2 (X = Cl, Br, I) gave 3,4-trans haloalkenyl five-membered heterocycles stereoselectively. The stereochemistry was determined by NOE experiments and reduction of the cyclized products. Various transformations of the haloalkenyl functionalized cyclic compounds have also been performed.
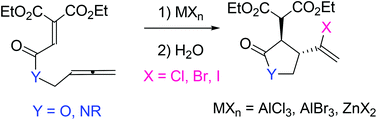

 Please wait while we load your content...
Please wait while we load your content...