Tuning the chiroptical and morphological properties of steroidal-porphyrin aggregates: a mechanistic, structural, and MM investigation†
Abstract
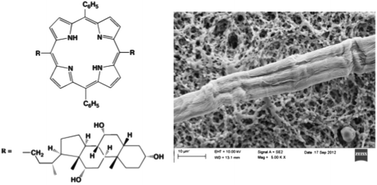
The aggregation of a steroid-functionalised porphyrin derivative occurs with the formation of J-type chiral species. Spectroscopic and SEM studies indicate that the initial concentration of the macrocycle strongly influences the morphology of the final mesoscopic structures, as a consequence of a change in the mechanistic course of the self-assembly process. Fibrillar structures are obtained at low porphyrin concentration, whereas aggregates of globular shapes are formed on increasing the substrate concentration. Molecular mechanics investigations gave insights into the intimate nature of the driving forces that govern the self-assembly process, pointing out the importance of ring distortion, of intramolecular steroidal OH–π hydrogen bonds, as well as dispersion forces among the tetrapyrrolic platforms.

 Please wait while we load your content...
Please wait while we load your content...