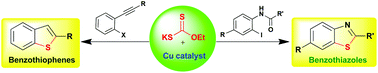
Cu-catalyzed in situ generation of thiol using xanthate as a thiol surrogate for the one-pot synthesis of benzothiazoles and benzothiophenes†
Abstract
A new copper-catalyzed in situ generation of aryl thiolates strategy was successfully developed for the one-pot synthesis of substituted

 Please wait while we load your content...
Please wait while we load your content...