An efficient synthesis of heptaaryldipyrromethenes from tetraarylcyclopentadienones and ammonium acetate and their extension to the corresponding BODIPYs†
Abstract
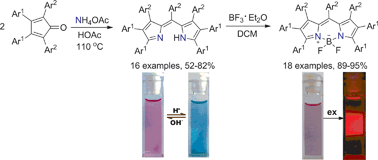
Heptaaryldipyrromethenes are efficiently prepared from ammonium acetate and tetraarylcyclopentadienones in a one-pot cascade process and can be converted into heptaaryl BODIPYs with fluorescent response to environmental acidity.

 Please wait while we load your content...
Please wait while we load your content...