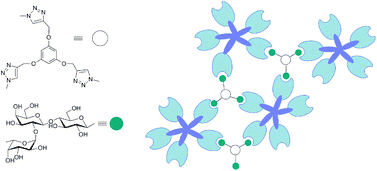
The emerging functional versatility of cellular glycans makes research on the design of synthetic inhibitors a timely topic. In detail, the combination of ligand (or headgroup or contact site) structure with spatial parameters that depend on topological and geometrical factors underlies the physiological selectivity of glycan-protein (lectin) recognition. We herein tested a panel of bi-, tri- and tetravalent compounds against two plant agglutinins and adhesion/growth-regulatory lectins (galectins). In addition, we examined the impact of headgroup tailoring (converting lactose to 2′-fucosyllactose) in combination with valency increase in two assay types of increasing biorelevance (from solid-phase binding to cell binding). Compounds were prepared using copper-catalysed azide alkyne cycloaddition from peracetylated lactosyl or 2′-fucosyllactosyl azides. Significant inhibition was achieved for the plant toxin with a tetravalent compound. Different levels of sensitivity were noted for the three groups of the galectin family. The headgroup extension to 2′-fucosyllactose led to a selectivity gain, especially for the chimera-type galectin-3. Valency increase established discrimination against the homodimeric proteins, whereas the combination of valency with the headgroup extension led to discrimination against the tandem-repeat-type galectin-8 for chicken galectins but not human galectins-3 and -4. Thus, detailed structure–activity profiling of glycoclusters combined with suitably modifying the contact site for the targeted lectin will help minimize cross-reactivity among this class of closely related proteins.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...