Triclorosilane-mediated stereoselective synthesis of β-amino esters and their conversion to highly enantiomerically enriched β-lactams†
Abstract
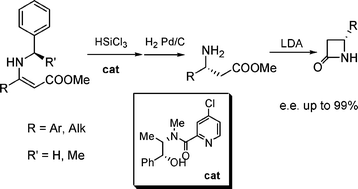
A highly stereoselective trichlorosilane-mediated reduction of N-benzyl enamines was developed; the combination of a low cost, easy to make metal-free

 Please wait while we load your content...
Please wait while we load your content...