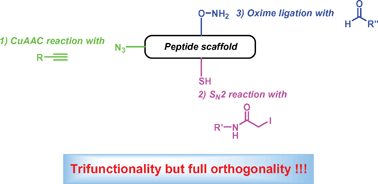
We describe for the first time, the synthesis and some bioconjugation applications of an original heterotrifunctional cross-linking reagent (also named tripod) bearing three different bioorthogonal functional groups which are fully compatible amongst themselves. Contrary to the first generation tripod recently reported by us (Org. Biomol. Chem., 2008, 6, 3065), the use of an azido group instead of the nucleophile-sensitive active carbamate moiety enables us to reach the targeted chemical orthogonality without the use of temporary aminooxy- and thiol protecting groups. Thus, the preparation of sophisticated bioconjugates through the sequential derivatisation of the tripod by means of copper-mediated 1,3-dipolar cycloaddition, oxime ligation and aqueous compatible mild thiol-alkylation reactions, is significantly simpler and more convenient. The chemoselective bioconjugation protocols were optimised through the preparation of FRET cassettes based on cyanine and/or xanthene fluorescent dye pairs and subsequent anchoring to fragile biomolecules. The applicability of this universal cross-linking reagent was also illustrated by the preparation of biochips suitable for aflatoxin B1 detection through the SPIT-FRI method.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...