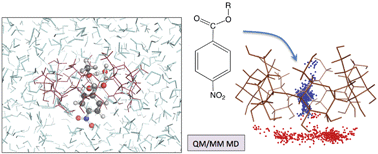
Cyclodextrins have attracted much interest in recent years because of their potential use as molecular reactors allowing organic reactions in aqueous solution. To better understand their effect on reaction mechanisms, we have carried out a computational study of a prototypical process (neutral ester hydrolysis) in a β-cyclodextrin (β-CD). Two models have been used for the reactor. The first and simpler one assumes that the medium can be described by a polarizable dielectric continuum. The second one takes into account the discrete nature of the β-CD and water molecules thanks to a computational approach that combines the use of Quantum Mechanics, Molecular Mechanics and Molecular Dynamics techniques. We focus on neutral pH processes for which either acceleration or inhibition has experimentally been observed depending on ester derivatives. Our calculations rationalize such observations by showing that the two reaction mechanisms usually invoked for hydrolysis, stepwise (involving two transitions states with formation of a –C(OH)2OR tetrahedral intermediate) and concerted, undergo opposite effects in the β-CD environment. The results highlight the role played by molecular shape recognition. Thus, in spite of a higher polarity exhibited by the three transition states with respect to the reactants, the interactions with the β-CD cavity may either increase or decrease the activation barrier due to different 3D-arrangements of the chemical structures.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...