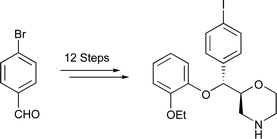
With the aim of developing a new SPECT imaging agent for the noradrenaline transporter, a twelve-step stereoselective synthesis of iodinated analogues of (2S,3R)- and (2R,3S)-reboxetine has been achieved from 4-bromobenzaldehyde. The key steps involve a Sharpless asymmetric epoxidation to establish the stereogenic centres and a copper catalysed aromatic halogen exchange reaction to introduce the key iodine atom. In vitro testing of these compounds using a [3H]nisoxetine displacement assay with homogenised rat brain shows both compounds to have significant affinity, with Ki values of 320.8 nM and 58.2 nM for (2S,3R)- and (2R,3S)-iodoreboxetine respectively.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...