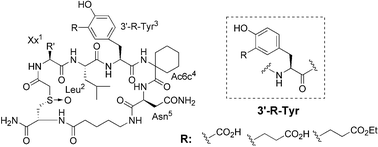
A new class of phosphotyrosyl (pTyr) mimetics, distinct from the conventional pTyr mimetic design of adding non-hydrolyzable acidic functionalities to the 4′-position of phenylalanine, was created by introducing carboxy-containing groups to the 3′-position of tyrosine. The effect of the chain length of the carboxy substituent was examined. Reported herein is the chiral pool synthesis of the new pTyr mimetics, and their first use in a novel non-phosphorylated Grb2–SH2 domain binding motif with the 5-amino-acid sequence Xx1-Leu-(3′-substituted-Tyr)-Ac6c-Asn. The highest affinity was exhibited by the 3-L-(2-carboxyethyl)tyrosine-containing sulfoxide-cyclized peptide 3c, with an IC50 = 1.1 µM, providing a promising new template for further development of potent Grb2–SH2 domain inhibitors with reduced charge and peptidic nature, but improved selectivity and bioavailability.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...