A computational study on the amine-oxidation mechanism of monoamine oxidase: Insight into the polar nucleophilic mechanism†
Abstract
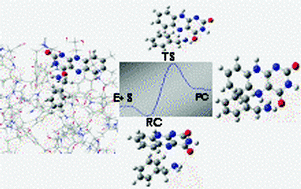
The proposed polar nucleophilic mechanism of MAO was investigated using quantum chemical calculations employing the semi-empirical PM3 method. In order to mimic the reaction at the enzyme's active site, the reactions between the

 Please wait while we load your content...
Please wait while we load your content...