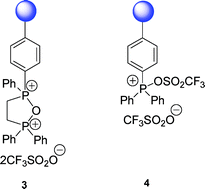
Two novel dehydrating reagents 3 and 4, based on a phosphonium anhydride and an oxyphosphonium triflate respectively, were prepared by reaction of the corresponding polymer-supported phosphine oxides with triflic anhydride. Reagent 3, based on the novel phosphorus heterocycle 1,1,3,3-tetraphenyl-2-oxa-1,3-diphospholanium bis(trifluoromethanesulfonate), was found to be a useful reagent for ester and amide formation. A wide range ofcoupling/dehydration-type reactions, such as ester, amide, anhydride, peptide, ether and nitrile formation, were performed in high yield using the more readily prepared polymer-supported triphenylphosphine ditriflate 4, which was easily recovered and re-used several times without loss of efficiency. With primary alcohols, both reagents 3 and 4 provide an alternative to the Mitsunobu reaction, where the use of azodicarboxylates and chromatography to remove the phosphine oxide by-product can be avoided. The use of 4-dimethylaminopyridine allowed the esterification of secondary alcohols with 4 to proceed in high yield but with retention of configuration.

 Please wait while we load your content...
Please wait while we load your content...