Simplified dynemicin analogues: diastereoselective synthesis and evaluation of their activity against plasmid DNA
Abstract
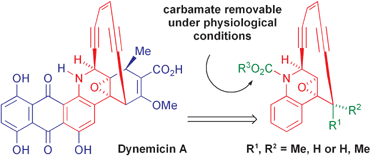
The total synthesis of two diastereoisomeric simplified dynemicin analogues is reported. The key steps involved are: the regio- and diastereoselective functionalisation of an appropriate racemic quinoline precursor and the ring closure to give the 10-membered enediyne moiety through a Pd(0)-catalysed Stille reaction. After the successful conversion of one of these derivatives into a compound more readily activable under nearly physiological conditions, the activity against plasmid DNA was evaluated.

 Please wait while we load your content...
Please wait while we load your content...