Synthesis, and the optical and electrochemical properties of a series of push–pull dyes based on the 4-(9-ethyl-9H-carbazol-3-yl)-4-phenylbuta-1,3-dienyl donor†
Abstract
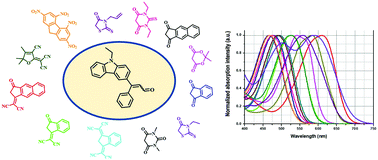
A series of twelve dyes based on the 4-(9-ethyl-9H-carbazol-3-yl)-4-phenylbuta-1,3-dienyl donor were prepared with electron acceptors varying in their structures but also in their electron-withdrawing ability. For specificity, a butadienyl spacer was introduced between the donor and the acceptor to both lower the bandgap and furnish dyes with high molar extinction coefficients. The different dyes A–N were characterized using various techniques including UV-visible absorption and fluorescence spectroscopy, and cyclic voltammetry. All dyes showed an intense intramolecular charge transfer band located in the visible range. To further investigate the optical properties of the twelve dyes, their solvatochromism was investigated in twenty-three solvents of different natures, enabling linear correlations to be obtained on different polarity scales such as the Taft, Reichardt and Catalan scales. To support the experimental results, the optical properties were compared with those theoretically determined.


 Please wait while we load your content...
Please wait while we load your content...