Bulk gold catalyzes hydride transfer in the Cannizzaro and related reactions†
Abstract
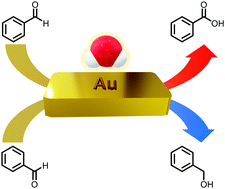
Bulk gold was found to catalyze the Cannizzaro reaction of benzaldehyde and related disproportionation reactions in superheated water. At 200 °C and 1.5 MPa for ∼48 hours, benzaldehyde conversions increased linearly with added gold at low loadings, but this trend reversed at higher gold loadings. Ratios of the primary products benzoic acid and benzyl alcohol exceeded unity, increasing with increasing amounts of gold. These observations are attributed to the reactivity of benzyl alcohol in the presence of gold, which is proposed to react via cross-disproportionation with benzaldehyde to yield toluene and benzoic acid, transitioning to benzyl alcohol disproportionation that forms toluene and reforms benzaldehyde at higher gold loadings. Turnover frequencies for both benzaldehyde (0.00013 s−1) and benzyl alcohol (0.0011 s−1) were quite low and experiments of varied duration demonstrated that very few turnovers occurred under the reaction conditions. Proposed mechanisms for the catalyzed reactions include hydride transfer from gold-bonded alkoxides to surface gold atoms. These observations expand the known catalytic capabilities of bulk gold to include hydride transfer, a fundamental process in organic chemistry and biochemistry. The simple, environmentally benign reaction conditions are reminiscent of organic geochemical processes, with implications for both geomimicry and green chemistry approaches to chemical challenges.


 Please wait while we load your content...
Please wait while we load your content...
