Insights into the mechanisms of Cu(i)-catalyzed heterocyclization of α-acyl-α-alkynyl ketene dithioacetals to form 3-cyanofurans: the roles of NH4OAc†
Abstract
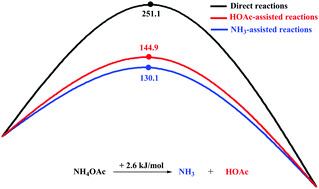
A computational study with the M06-2X/B3LYP density functional is carried out to explore the effects of NH4OAc on the Cu(I)-catalyzed heterocyclization of α-acyl-α-alkynyl ketene dithioacetals to form 3-cyanofurans. The calculations suggest the following. (a) The use of CuBr as the catalyst decreases significantly the free energy barriers of intramolecular cyclization and intermolecular addition. (b) NH4OAc can be decomposed into NH3 and HOAc. NH3 as the proton shuttle is critical in the stepwise H+-transport process because it can greatly reduce the rate-determining free energy barrier of whole Cu(I)-catalyzed reactions (from 251.1 to 130.1 kJ mol−1). (c) HOAc also plays a role similar to that of NH3 in the H+-transport reactions. These studies are expected to improve our understanding of transition-metal-catalyzed reactions and provide guidance for the future design of new catalysts and new reactions.


 Please wait while we load your content...
Please wait while we load your content...