Selective photocatalytic C–C coupling of isopropanol into pinacol with concurrent hydrogen evolution over GONaOH photocatalyst†
Abstract
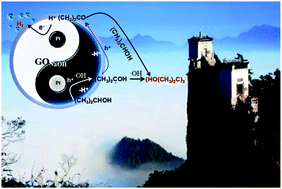
If a sacrificial agent is selectively oxidized to generate high-value chemicals during photocatalytic hydrogen production, then photocatalytic water splitting is more meaningful in consideration of green chemistry. GONaOH was obtained after hydrothermal treatment of graphene oxide (GO) using 10 mol L−1 NaOH. The average hydrogen production rate could reach 7.36 mmol h−1 over 0.50 g L−1 GONaOH photocatalyst for a reaction of 12 h. And the sacrificial agent isopropanol could undergo selective oxidative C–C coupling to generate pinacol. The isopropanol conversion rate was 77.95%, and the pinacol selectivity was 62.32%. Also, the GONaOH photocatalytic selective oxidative C–C coupling mechanism of isopropanol was further studied. The ˙OH concentration on the photocatalyst surface was verified as a key factor influencing pinacol selectivity. Under the effect of GONaOH photocatalyst, ˙OH radicals were used for the oxidative dehydrogenation of isopropanol to generate 2-hydroxyisopropyl radicals, and pinacol was generated through C–C coupling. Meanwhile, acetone byproducts could further undergo hydrogenation–dehydrogenation coupling reaction with isopropanol to finally generate pinacol.


 Please wait while we load your content...
Please wait while we load your content...