The catalyst free synthesis of α-fluorinated aroylacyl imides†
Abstract
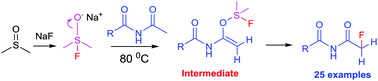
The first approach to α-fluorination of aroylacyl imides without a catalyst was accomplished, relying on electrophilic activation through the reaction between a fluoride salt, dimethylsulfoxide and an imide. The synthesis of α-fluorinated aroylacyl imides in good yields was achieved using inexpensive reagents such as NaF and DMSO.

 Please wait while we load your content...
Please wait while we load your content...