A 2-((4-Arylpiperazin-1-yl)methyl)phenol ligated Pd(ii) complex: an efficient, versatile catalyst for Suzuki–Miyaura cross-coupling reactions†
Abstract
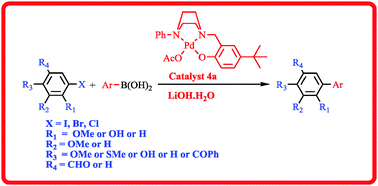
N,N,O-Tridentate palladium(II) complexes [Pd(OAc){R-C4H8N2(CH2Ar)}] (R = Ph, Ar = 4-tBu-C6H3–OH (4a)) and [2,4-di-tBu-C6H2–OH, R = benzyl (4b)] 4a and 4b have been synthesized from the corresponding 2-((4-arylpiperazin-1-yl)methyl)phenol ligands 3a and 3b in quantitative yields. The synthesized ligands and their palladium(II) complexes were characterized by NMR, IR and HRMS analyses. Complex 4a has been used as an efficient catalyst for the Suzuki cross-coupling reaction of 5-iodovanillin and 5-bromosalicylaldehyde with various arylboronic acids in low catalytic amounts (0.01–0.05 mol% of 4a). Moreover, this catalytic system is even applicable for Suzuki coupling reactions of deactivated aryl bromides and aryl chlorides, affording the cross-coupling products in good to excellent yields with a broad substrate scope.

 Please wait while we load your content...
Please wait while we load your content...